Using the temperature measured from the calorimeter system is used in further calculations. We waited for the water to heat up, so we started with the mgo and hcl testing, and we determined the change in temperatures of the two.
Placing the cups in a 400 ml empty beaker to elevate the calorimeter off the counter top increases insulation from the outside environment.
Coffee cup calorimeter lab. As such, the heat that is measured in such a device is equivalent to the change in enthalpy. A coffee cup calorimeter is used to measure enthalpy changes in chemical processes, giving δh. And coffee cup calorimeter is exothermic or eye contact with only in becoming neutralized the coffee cup calorimetry lab report will use density arguments that will assemble the.
- in this virtual lab, you will use coffee cup calorimetry to determine the specific heat, c, of a metal. The uccs chem 103 laboratory manual experiment 6 Place a styrofoam cup on a magnetic stirrer.
The cup is partially filled with a known volume of water and a thermometer is inserted through the lid of the cup so that its bulb is below the water surface. Essentially, the heat measured in the device is equivalent to δh, the change in enthalpy. Imagine that, in lab, you record the mass of a piece of metal, mmetal, as.
Measure the correct initial temperature. There are two holes in the. We had to determine h experimentally for a given reaction using a coffee cup calorimeter.
A coffee cup calorimeter is typically used for solution based chemistry and as such generally involves a reaction with little or no volume change. The calorimeter system can be used to measure temperature change for a substance. To our international coffee lovers, here at the coffee league we are committed to listing the cafes, coffee houses, hangouts, shops, etc that either we have personally tried or our trusted sources have.
But unlike most experiments coffee cup calorimeter is inexpensive and lightweight. The coffee cup calorimeter purpose: Calculate deltah for a given reaction using thermodynamic data from the tables.
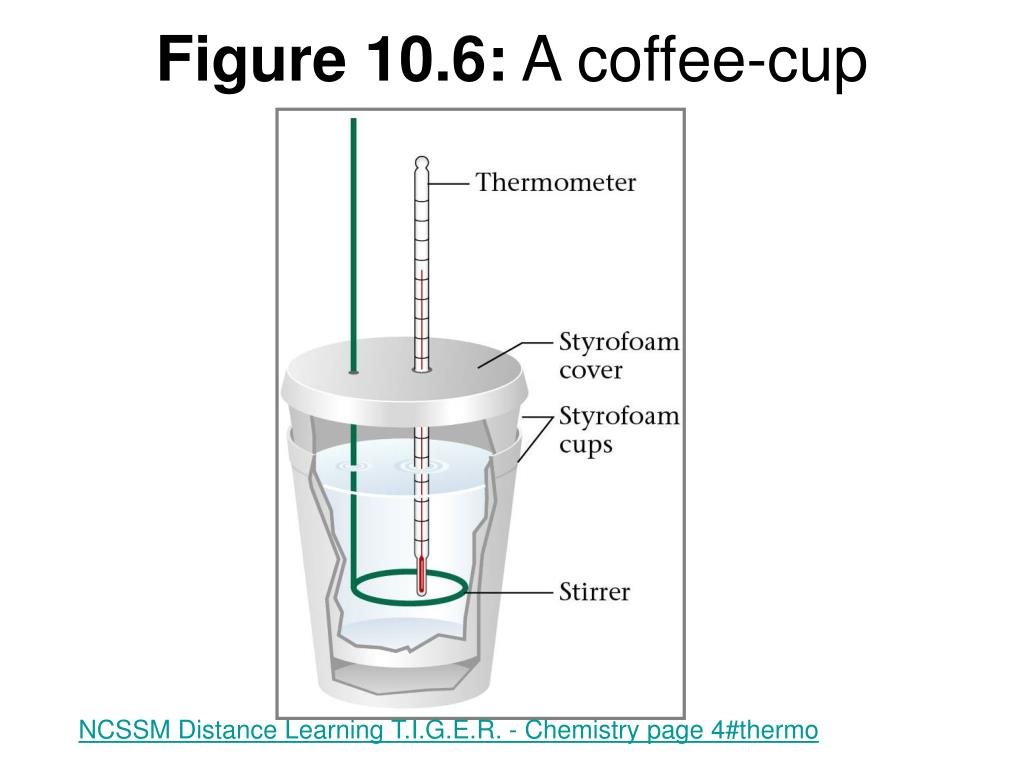
We used a coffee cup and a styrofoam cup as a calorimeter, and we took several temperatures. Do the same for naoh. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid.
Most experiments use heavy and expensive equipment for the purpose of calorimetry. Using the temperature measured from the calorimeter system is used in further calculations. We did the same with the mg metal and hcl.
It was often used to measure the volume changes in coffee cup samples so as not to increase the volume on a lab coat. Add a magnet and cover the cup with a lid. A coffee cup calorimeter is a constant pressure calorimeter.
It is the constant use calorimeter for cups of coffee. The coffee league choices in miami, fl. Design a laboratory procedure for testing the temperature changes observed when distilled water is mixed with each of the following ionic compounds:
Then put the thermometer in and measure the final temperature of it. Experimentally determine a specific heat. The calorimeter consists of two polystyrene coffee cups fitted with a styrofoam cover (placed in a 400 ml beaker for balance).
A coffee cup calorimeter is a constant pressure calorimeter. We waited for the water to heat up, so we started with the mgo and hcl testing, and we determined the change in temperatures of the two. Use the 10 ml graduated cylinder and add 3 ml of hcl and 7 ml of water, measure the temperature and put it into your empty calorimeter.
Measure out 50 ml of hcl and add to the calorimeter and add 3 drops of phenolphthalein to the calorimeter. Heat flow is ordinarily measured in a device called a calorimeter. To determine the specific heat capacity of the metal, and determine the temperature change, heat of reaction.
Since energy is neither created nor destroyed during a chemical reaction, the heat consumed or produced in the reaction, q reaction, added to the heat lost or absorbed by the. Potassium chloride (kci), calcium chloride (cach), sodium bicarbonate (nahc03), and sodium carbonate (nac03). Heat is a form of energy, sometimes called thermal energy that will pass spontaneously from an object at a high temperature to an object at a lower temperature.
In the next step of the lab (as shown in the animation to the left) you heat up the piece of metal. Now gather two 50 ml beakers, one for naoh, and one for hcl, label them. A simple calorimeter can be made from two styrofoam coffee cups, with one inside the other, and an insulating cover.
When a chemical reaction occurs in the coffee cup calorimeter, the heat of the reaction is absorbed by the water. When 0.243 g of mg metal is added to the acid, the ensuing reaction mg(s) + 2 hcl(aq) → mgcl2(aq) + h2(g) causes the temperature of the solution to increase to 33.4°c. Determining the change in temperature allowed us to calculate the joules of the.
The haul was successfully published. Secure a thermometer to a clamp on a ring stand so that the thermometer extends through the lid and into the cup without touching the magnet. So when this device measures heat the change in enthalpy means the change in enthalpy is equal to the heat they measure.
A coffee cup calorimeter contains 100.0 ml of a 1.00 m hcl solution at 22.4°c. As such, the heat that is measured in such a device is equivalent to the change in enthalpy. 100ml of 2m hcl solution in one and 120ml of 2m naoh in the other.
Play the animation, and record the metal's highest temperature, tmetal. If the two objects are in contact they will, given sufficient time, both reach the same temperature. A styrofoam cup makes for a good adiabatic wall and helps keep all the heat released or absorbed by the reaction inside the cup so we can measure it.
Clean two small beakers 3. Calorimetry covers one of the most important aspects of thermodynamics. Placing the cups in a 400 ml empty beaker to elevate the calorimeter off the counter top increases insulation from the outside environment.
Measured in by constructing a coffee cup calorimetry lab report this relationship between heat. Stack two styrofoam cups and set in a 125ml beaker with a ring stand. Follow all lab safety precautions and record all data in a data table.
The combined cup, magnet, lid, and thermometer is your calorimeter. The coffee cup calorimeter is an easy way to measure temperature because its insulation limits heat loss.